name the ester made from ethanol and ethanoic acid An introduction to esters
An introduction to esters:
Image 1
 Esters are a fascinating group of organic compounds that have a wide range of uses in various industries including food, pharmaceuticals, and cosmetics. They are formed through a chemical reaction between an alcohol and an organic acid, resulting in the formation of an ester and water. This process is known as esterification.
Esters are a fascinating group of organic compounds that have a wide range of uses in various industries including food, pharmaceuticals, and cosmetics. They are formed through a chemical reaction between an alcohol and an organic acid, resulting in the formation of an ester and water. This process is known as esterification.
Esters are known for their pleasant and distinctive smells, which are often used in perfumes and flavorings. One of the most well-known examples of an ester is isoamyl acetate, which gives bananas their characteristic scent. This compound is also used in the production of artificial banana flavorings.
The structure of an ester consists of a carbonyl group, which is a carbon atom double-bonded to an oxygen atom, and an alkyl or aryl group. The alkyl or aryl group determines the specific properties and uses of the ester. For example, methyl salicylate, which is an ester with a methyl group, is used in topical analgesic creams for its pain-relieving properties.
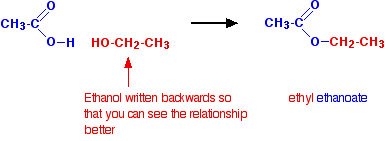
Image 2
 Now, let’s test your knowledge on esters. Take a look at the image above, which shows a chemical reaction between an alcohol and an organic acid. Your task is to choose the name of the ester that would result from this reaction.
Now, let’s test your knowledge on esters. Take a look at the image above, which shows a chemical reaction between an alcohol and an organic acid. Your task is to choose the name of the ester that would result from this reaction.
The ester formed in this reaction is ethyl ethanoate. This compound is commonly known as ethyl acetate and is widely used as a solvent in various industries. It has a fruity odor and is commonly found in nail polish removers, adhesives, and paints.
Esters play a crucial role in the field of organic chemistry as they are involved in various reactions such as hydrolysis, saponification, and transesterification. Additionally, they are used as intermediates in the synthesis of numerous organic compounds.
In conclusion, esters are versatile compounds with a wide range of applications. From giving fruits their pleasant aromas to being used as solvents and flavorings, esters are vital in many aspects of our daily lives. Understanding their properties and reactions allows scientists to harness their potential for various industrial purposes.
If you are looking for an introduction to esters you’ve visit to the right page. We have 5 Pictures about an introduction to esters like [Chemistry] Differentiate between Ethanol and Ethanoic acid - Class 10, IGCSE Chemistry 2017: 4.39C: Know that Ethyl Ethanoate is the Ester and also [Chemistry] Differentiate between Ethanol and Ethanoic acid - Class 10. Read more:
An Introduction To Esters
 www.chemguide.ukesters acid ester ethanol ethanoic equation water properties between diagram chemguide organic chemistry intended isn libretexts fats gif organicprops
www.chemguide.ukesters acid ester ethanol ethanoic equation water properties between diagram chemguide organic chemistry intended isn libretexts fats gif organicprops
Carbon And Its Compounds
 transitiononweb.blogspot.comacid reaction ester form base ethanoic class reacts its 10th acetic strong used smell
transitiononweb.blogspot.comacid reaction ester form base ethanoic class reacts its 10th acetic strong used smell
IGCSE Chemistry 2017: 4.39C: Know That Ethyl Ethanoate Is The Ester
igcse-chemistry-2017.blogspot.comacid ethanoate ethyl sulfuric ethanoic ester esters bbc chemistry organic ethanol reaction water gcse formation reacts diagram sodium presence chemical
Choose The Name Of The Ester That Would Result From The Reaction
 zuoti.pro[Chemistry] Differentiate Between Ethanol And Ethanoic Acid - Class 10
zuoti.pro[Chemistry] Differentiate Between Ethanol And Ethanoic Acid - Class 10
![[Chemistry] Differentiate between Ethanol and Ethanoic acid - Class 10](https://d1avenlh0i1xmr.cloudfront.net/8b2659af-0499-4edb-aff1-c5a5a8beec2f/ethanol-vs-ethanoic-acid---teachoo.jpg) www.teachoo.comCarbon and its compounds. [chemistry] differentiate between ethanol and ethanoic acid. Acid reaction ester form base ethanoic class reacts its 10th acetic strong used smell
www.teachoo.comCarbon and its compounds. [chemistry] differentiate between ethanol and ethanoic acid. Acid reaction ester form base ethanoic class reacts its 10th acetic strong used smell